41 fda approved health claims on food labels
Health Claims on Food Labels - LabelCalc Health claims, according to the FDA, are statements about the relationship between a food product or ingredient and a reduced risk of disease or a health condition. Basically, the FDA distinguishes two kinds of health claims: "authorized" and "qualified." Authorized Health Claims: Claims that have significant scientific agreement (SSA ... Authorized Health Claims That Meet Significant Scientific Agreement Authorized Health Claims That Meet the Significant Scientific Agreement (SSA) Standard Authorized health claims in food labeling are claims that have been reviewed by FDA and are allowed on food...
Questions and Answers on Health Claims in Food Labeling | FDA All health claims, whether authorized or qualified, require pre-market review by the FDA. Under federal law, the FDA approves by regulation authorized health claims for use in food labeling only if...

Fda approved health claims on food labels
A Guide to FDA Regulation of Food Labeling Claims Among the FDA-regulated claims commonly declared on food labels are nutrient-content claims, health claims, qualified health claims and structure/function claims. Additionally, FDA has authority over claims related to gluten content, genetically modified organisms (GMOs) and "natural." Introduction to Food Product Claims — FDA Reader A Qualified Health Claim is a statement approved by the FDA for use on food labels that has strict wording requirements. When there is emerging evidence between a food and the reduced risk of a disease or health condition, but not enough for the FDA to issue an Authorized Health Claim, the FDA may approve a "Qualified Health Claim". Authorized Health Claims That Meet Significant Scientific Agreement Authorized Health Claims That Meet the Significant Scientific Agreement (SSA) Standard Authorized health claims in food labeling are claims that have been reviewed by FDA and are allowed on food...
Fda approved health claims on food labels. The FDA Wants to Update the Definition for "Healthy" Claims on Food Labels The FDA is looking to regulate the use of the word because of the rise of diet-related illnesses in the U.S. "In the current marketplace, about 5 percent of all packaged foods are labeled as 'healthy,'" the FDA writes in the proposal. "Because nutrition science has evolved over time, updating the definition of the implied nutrient content claim ... › regulatory-information › search-fdaSmall Entity Compliance Guide on Structure/Function Claims | FDA On January 6, 2000, the Food and Drug Administration (FDA) published a final rule in the Federal Register defining the types of statements that may be used on the label and in the labeling of ... Health Claims on Food Labels - Consumer Reports Specifically, grass-fed meat and dairy has a more healthful ratio of omega-6 polyunsaturated fatty acids to omega-3s. Too much omega-6 fat in your diet can cause inflammation, which may be a ... FDA Proposes Updated Definition of 'Healthy' Claim on Food Packages to ... FDA Proposes Updated Definition of 'Healthy' Claim on Food Packages to Help Improve Diet, Reduce Chronic Disease For Immediate Release: September 28, 2022 Today, the U.S. Food and Drug...
Label Claims for Conventional Foods and Dietary Supplements there are three ways in which fda exercises its oversight in determining which health claims may be used on a label or in labeling for a conventional food or dietary supplement: 1) the 1990... en.wikipedia.org › wiki › Food_and_Drug_AdministrationFood and Drug Administration - Wikipedia The United States Food and Drug Administration (FDA or USFDA) is a federal agency of the Department of Health and Human Services.The FDA is responsible for protecting and promoting public health through the control and supervision of food safety, tobacco products, dietary supplements, prescription and over-the-counter pharmaceutical drugs (medications), vaccines, biopharmaceuticals, blood ... › it-really-fda-approvedIs It Really 'FDA Approved'? | FDA - U.S. Food and Drug ... May 10, 2022 · The FDA is responsible for protecting public health by regulating human drugs and biological products, animal drugs, medical devices, tobacco products, food (including animal food), cosmetics, and ... › food › food-labeling-nutritionChanges to the Nutrition Facts Label | FDA - U.S. Food and ... Mar 07, 2022 · Manufacturers with $10 million or more in annual sales were required to update their labels by January 1, 2020; manufacturers with less than $10 million in annual food sales were required to ...
Label Claims for Food & Dietary Supplements | FDA Among the claims that can be used on food and dietary supplement labels are three categories of claims that are defined by statute and/or FDA regulations: health claims, nutrient content claims,... Qualified Health Claims — FDA Reader A Qualified Health Claim is a statement approved by the FDA for use on food labels that has strict wording requirements. When there is emerging evidence between a food and the reduced risk of a disease or health condition, but not enough for the FDA to issue an Authorized Health Claim, the FDA may approve a "Qualified Health Claim" Questions and Answers on Health Claims in Food Labeling | FDA Health claims in food labeling are claims that have been reviewed by FDA and are allowed on food products to show that a food or food component may reduce the risk of a disease or a health-related condition. ... To be approved by the FDA as an authorized health claim, there must be significant scientific agreement (SSA) among qualified experts ... › food › food-industryHow to Start a Food Business | FDA - U.S. Food and Drug ... Food Businesses Subject to FDA Regulation. FDA regulates all foods and food ingredients introduced into or offered for sale in interstate commerce, with the exception of meat, poultry, and certain ...
› pet-food-labels-generalPet Food Labels - General | FDA Pet food labeling is regulated at two levels. The federal regulations, enforced by the United States Food and Drug Administration (FDA), establish standards applicable for all animal feeds: proper ...
Food Packaging Claims | American Heart Association There are three categories of claims defined by statute and/or FDA regulations that can be used on food and dietary supplement labels: health claims, nutrient content claims, and; structure/function claims. A "health claim" by definition has two essential components: A substance (whether a food, food component, or dietary ingredient) and
Label Claims for Conventional Foods and Dietary Supplements there are three ways in which fda exercises its oversight in determining which health claims may be used on a label or in labeling for a conventional food or dietary supplement: 1) the 1990...
Qualified Health Claims | FDA - U.S. Food and Drug Administration Food manufacturers can petition the agency to consider exercising enforcement discretion for the use of a qualified health claim. The FDA does not "approve" qualified health claim petitions.
FDA Label Search - Food and Drug Administration The drug labels and other drug-specific information on this Web site represent the most recent drug listing information companies have submitted to the Food and Drug Administration (FDA). (See 21 CFR part 207.) The drug labeling and other information has been reformatted to make it easier to read but its content has neither been altered nor ...
Label Claims for Food & Dietary Supplements | FDA Among the claims that can be used on food and dietary supplement labels are three categories of claims that are defined by statute and/or FDA regulations: health claims, nutrient content claims,...
Nutrient Content Claims | FDA - U.S. Food and Drug Administration Nutrient Content Claims. See Claims That Can Be Made for Conventional Foods and Dietary Supplements for definitions of claims. Final Rule: Food Labeling: Nutrient Content Claims; Alpha-Linolenic ...
› news-events › public-health-focusWarning Letters and Test Results for Cannabidiol-Related ... May 06, 2022 · Contact FDA Follow FDA on Facebook Follow FDA on Twitter View FDA videos on YouTube Subscribe to FDA RSS feeds FDA Homepage Contact Number 1-888-INFO-FDA (1-888-463-6332)
FDA perspectives on health claims for food labels - PubMed Abstract. The U.S. Food and Drug Administration's regulatory authority over health claims was clarified in 1990 legislation known as the Nutrition Labeling and Education Act (NLEA). This law established mandatory nutrition labeling for most foods and placed restrictions on the use of food label claims characterizing the levels or health ...
Authorized Health Claims That Meet Significant Scientific Agreement Authorized Health Claims That Meet the Significant Scientific Agreement (SSA) Standard Authorized health claims in food labeling are claims that have been reviewed by FDA and are allowed on food...
Introduction to Food Product Claims — FDA Reader A Qualified Health Claim is a statement approved by the FDA for use on food labels that has strict wording requirements. When there is emerging evidence between a food and the reduced risk of a disease or health condition, but not enough for the FDA to issue an Authorized Health Claim, the FDA may approve a "Qualified Health Claim".
A Guide to FDA Regulation of Food Labeling Claims Among the FDA-regulated claims commonly declared on food labels are nutrient-content claims, health claims, qualified health claims and structure/function claims. Additionally, FDA has authority over claims related to gluten content, genetically modified organisms (GMOs) and "natural."




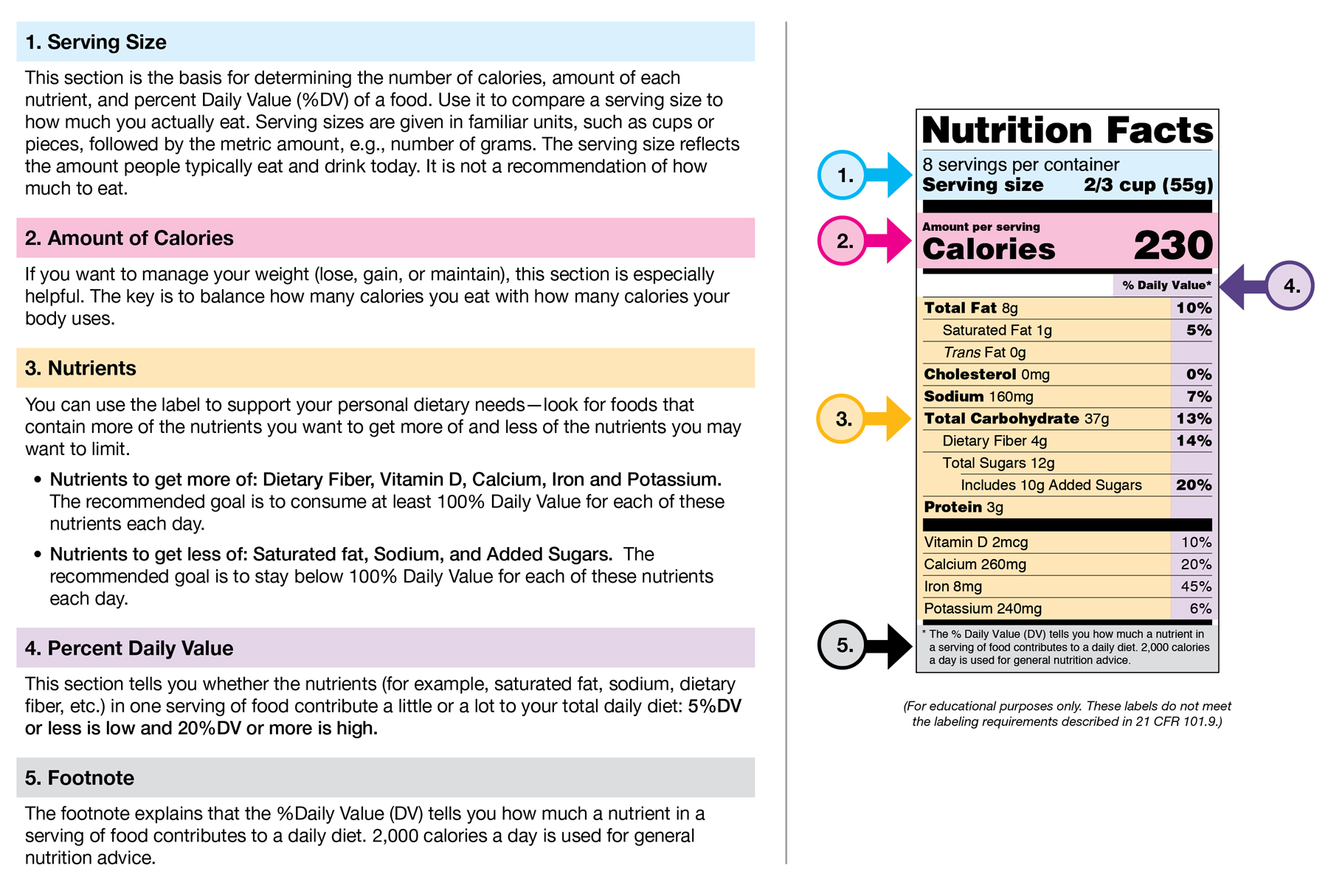






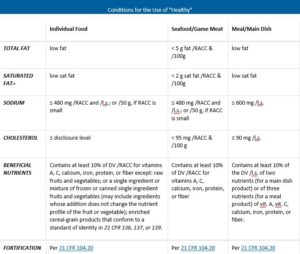








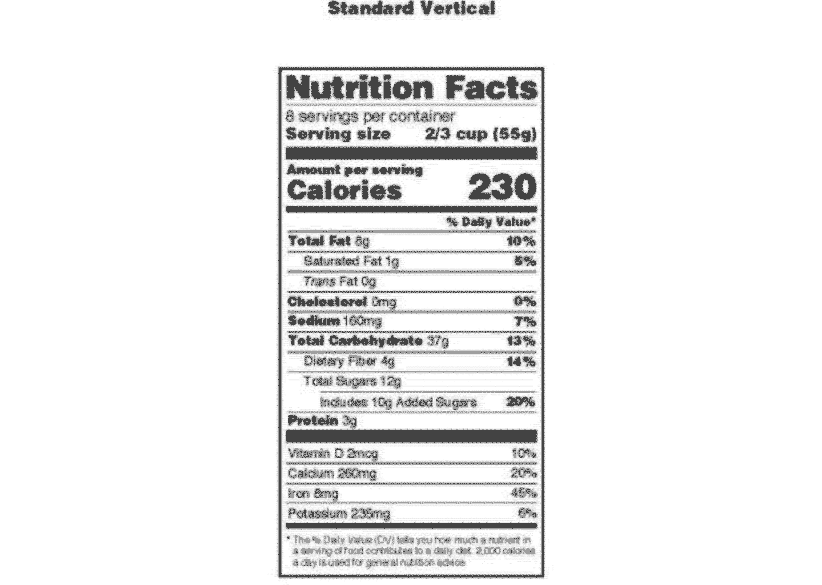











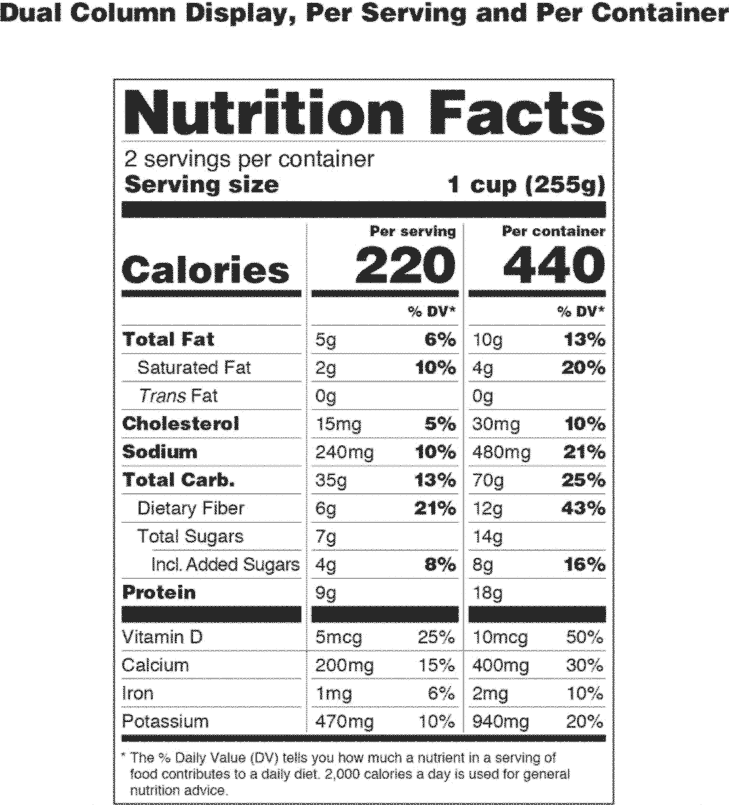
Post a Comment for "41 fda approved health claims on food labels"